lucas reagent|beilstein test : Cebu The Lucas reagent can be prepared by the following steps: 1. Pour the concentrated HCl into a 50 ml graduated cylinder. Measure out 47 ml of concentrated HCl and pour it into the 100 ml beaker 2. Place the 100 ml . Tingnan ang higit pa Du har søgt efter GIVER på 5 bogstaver og resultaterne ses nedenfor.. Vi fandt 60 løsningsforslag for ordet GIVER som du kan bruge til at løse din krydsord. Løsningen vil ofte været et synonym til GIVER.. Det er muligt at få mere information om de enkelte løsningsord ved at klikke på dem.The Whole Fairly Oddparents Franchise is owned by Nickelodeon. Addeddate 2023-07-31 04:15:03 Color color Identifier 0x-08-scouts-honor Scanner Internet Archive HTML5 Uploader 1.7.0 Sound sound Year 2017 . plus-circle Add Review. comment. Reviews Reviewer: MaddieL13 - .
lucas reagent,Lucas reagent is a solution of anhydrous zinc chloride (Lewis acid) in concentrated hydrochloric acid. It is used as a reagent to test alcohols and classify them in accordance to their reactivity. The reaction is a substitution reaction where the chloride of the zinc chloride gets replaced by the . Tingnan ang higit paThis test is more often used to categorize the different types of alcohols based on the time taken to form a turbid solution or precipitation using the Lucas Reagent namely: 1. . Tingnan ang higit paThe Lucas reagent can be prepared by the following steps: 1. Pour the concentrated HCl into a 50 ml graduated cylinder. Measure out 47 ml of concentrated HCl and pour it into the 100 ml beaker 2. Place the 100 ml . Tingnan ang higit paLucas reagent is highly toxic and corrosive and should be handled carefully while conducting the experiment. The toxicity and . Tingnan ang higit paThe reaction which normally occurs is a SN1 nucleophilic substitution which is a two steps reaction. Alcohols which have a capability to form carbocation intermediates exhibit this reaction. Only secondary . Tingnan ang higit pa
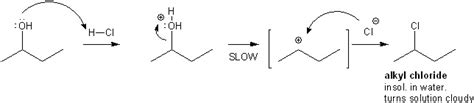
"Lucas' reagent" is a solution of anhydrous zinc chloride in concentrated hydrochloric acid. This solution is used to classify alcohols of low molecular weight. The reaction is a substitution in which the chloride replaces a hydroxyl group. A positive test is indicated by a change from clear and colourless to turbid, signalling formation of a chloroalkane. Also, the best results for this tes.
Learn how to use the Lucas reagent to identify primary, secondary and tertiary alcohols based on their reactivity and rate of formation of chloroalkanes. See the detailed .
Lucas reagent converts alcohols to alkyl chlorides: tertiary alcohols give an immediate reaction, indicated when the alcohol solution turns cloudy; secondary alcohols usually .
Lucas Test is a method to distinguish primary, secondary and tertiary alcohols using Lucas reagent, a solution of hydrochloric acid and zinc chloride. Learn the reaction .
Learn how to prepare and use Lucas' reagent, a chemical reagent that identifies alcohols by forming a chloroalkane. Find the original reference and a brief .Lucas reagent converts alcohols to alkyl chlorides: tertiary alcohols fastest followed by secondary alcohols; primary alcohols do not react to any significant extent. Thus, Lucas .
Lucas reagent for differentiation of different alcoholsPlease Subscribe to my channelSupport me on patreonhttps://www.patreon.com/vibzzlabOrSupport me via pa.
Learn how to use three chemical tests to identify unknown alcohols: ferric chloride for phenols, Jones' reagent for primary and secondary alcohols, and Lucas reagent for .
Lucas reagent converts alcohols to alkyl chlorides: tertiary alcohols give an immediate reaction, indicated when the alcohol solution turns cloudy; secondary alcohols usually show evidence of reacting within five .盧卡斯 報導以來 .
Add the ZnCl2 to the hydrochloric acid in the beaker slowly. Stir the mixture with a glass stirring rod until the ZnCl2 dissolves. Add the ZnCl2 slow to avoid the mixture overflowing the sides of the small beaker. Adding the solid too fast will cause the solution to foam. Pour the Lucas Reagent into the 150 ml brown storage bottle.lucas reagent beilstein testThe Lucas reagent is a solution of concentrated hydrochloric acid and anhydrous zinc chloride. Equimolar quantities of concentrated HCl and ZnCl 2 are taken to make the reagent. Lucas test for alcohols gives a positive indication when a clear and colourless characteristic of the reagent changes to a turbid, .卢卡斯报导以来,这个测试方法已经成为标准的鉴 . Lucas reagent for differentiation of different alcoholsPlease Subscribe to my channelSupport me on patreonhttps://www.patreon.com/vibzzlabOrSupport me via pa.

Lucas test is performed by following steps –. Preparation of Lucas Reagent – Take equimolar quantities of zinc chloride and concentrated HCl and make a solution. Take a very small quantity of the given sample in a test tube. Now add ~2ml of the Lucas reagent in the test tube containing the given sample and mix them. In this experiment, we will review the Lucas test for alcohols. This test depends on the appearance of an alkyl chloride as an insoluble second layer when a.
Lucas' reagent, which is a mixture of zinc chloride and hydrochloric acid, converts secondary and tertiary alcohols to chloroalkanes at room temperature. Chloroalkanes are nearly insoluble in water, so a positive result appears as the mixture separates into a cloudy chloroalkane-containing layer over a clear layer. A solution of zinc chloride in concentrated hydrochloric acid (Lucas reagent) is a convenient reagent to differentiate between primary, secondary, and tertiary alcohols with less than eight or so carbons. Tertiary alcohols react very rapidly to give an insoluble layer of alkyl chloride at room temperature. Secondary alcohols react in several .
beilstein testThese authors performed the Lucas test on a number of alcohols and discuss the problems associated with the Lucas test's detection of secondary alcohols. KEYWORDS (Audience): Second-Year Undergraduate

Figure 5-8. The Lucas test is a visual method to differentiate primary, secondary, and tertiary alcohols by their varying ease of conversion to chlorides. A separate layer of alkyl chloride is observed in a positive test. Reaction of t-butyl alcohol with HCl to produce water insoluble t-butyl chloride. Heating an alcohol with H 2 S0 4 results .Lucas-Probe. Die Lucas-Probe (auch Lucas-Test) ist eine Nachweisreaktion in der organischen Chemie zur Unterscheidung von primären, sekundären und tertiären Alkoholen. Dabei reagieren Alkohole abhängig von der Stellung der Hydroxygruppe im Molekül des Alkohols unter nucleophiler Substitution unterschiedlich schnell oder gar nicht. Synonyms. Lucas reagent. zinc chloride hydrogen chloride. CRUISIDZTHMGJT-UHFFFAOYSA-L. Molecular Weight. 172.7 g/mol. Computed by PubChem 2.1 (PubChem release 2021.05.07) .Tertiary alcohols do not react with Jones’ reagent because they are resistant to oxidation. Finally, we can distinguish aliphatic alcohols with the Lucas test. Lucas’ reagent, which is a mixture of zinc chloride and hydrochloric acid, reacts with secondary and tertiary alcohols through an S N 1 nucleophilic substitution reaction. The zinc .
lucas reagent This video explores the Lucas test and oxidation tests using acidified dichromate and permanganate solutions to distinguish between primary, secondary and te.
Thus. the reaction of alcohols with Lucas reagent (HCIZnCl2) is used as a test to distinguish between 1°,2° and 3° alcohols. This can be done by observing that. (i) in case of 3° alcohols, cloudiness appears immediately. (ii) in case of 2° alcohols, cloudiness appears after a few minutes. (ii) in case of 1° alcohols, no cloudiness appears.
Lucas Test for Primary, Secondary, and Tertiary Alcohols. 1-butanol, 2-butanol, and 2-methyl-2-propanol are treated with a solution of ZnCl 2 in concentrated aqueous HCl. Three test tubes contain a solution of zinc chloride (ZnCl 2) in concentrated aqueous hydrochloric acid (HCl). The primary alcohol 1-butanol, the secondary alcohol 2-butanol .Lucas Test For Alcohols. The Lucas test differentiates between primary, secondary and tertiary alcohols by using Lucas’ reagent: concentrated HCl, ZnCl 2 (catalyst). HCl causes substitution reaction with the hydroxyl functional group, resulting in the production of a halogenated hydrocarbon. The halogenated product has lower solubility in water.
lucas reagent|beilstein test
PH0 · lucas test
PH1 · beilstein test
PH2 · Iba pa
PH3 · 2 4 dnph test